1.1 INTRODUCTION
Water is so important in living organisms that it constitutes a large percentage of both the intracellular and extracellular fluids, (above 70%) Water is essential for solubilizing and modifying the properties of polar biomolecules such as nucleic acids, proteins, and carbohydrates. This it does by forming hydrogen bonds with their polar functional groups such as the carboxylic (-COOH), amino (-NH2), hydroxyl, (OH), aldehyde (-CHO) and carbonyl (-C=O ) functional groups. These hydrogen bond interactions bring about conformational changes of these biomolecules in the aqueous environment, and these changes are essential to their functionality and the life process as a whole.
Again the structural and functional stability of biomolecules depend on the pH of the intracellular or extracellular fluids. The dissociation behaviour of the functional groups of biomolecules depends on the pH of the medium. And this is turn affects the rate of the enzyme-catalyzed reactions in the cells. The extracellular fluids, e.g blood also depend on the pH to function in transporting nutrients, metabolic waste products, gases and hormones round the body.
Thus there is a pH optimum for optimal biologic function of both the intracellular and extracellular constituents of living organisms, that needs to be maintained. For the blood the pH for optimum activity is 7.4, and for the stomach gastric juice it is between 2.0 and 3.0. The maintenance of pH in living organisms at a constant optimum value is carried out by the buffer systems. In the blood, one of the most important of the blood buffers is the carbonic acid buffer system.
1.2 HOMEOSTASIS
This is the maintenance of the composition of the internal environment of
living things. It ensures that the right internal environment is preserved for the
right functioning of living organisms. Homeostatic regulation of the internal
environment ensures among other things that there is a balanced distribution
of water in the body, and that the appropriate pH of body fluids is maintained.
The regulation of water balance in the body depends on the following factors:
i. Hypothalamus (the part of the brain that regulates thirst).
ii. Antidiuretic hormone (ADH), vasopressin.
ANTIDIURETIC HORMONE (ADH)
Vasopressin in also called antidiuretic hormone. It increases water
reabsorption in the kidney
ii. Ability of the kidneys to retain or excrete water
iii. Evaporative losses of water due to respiration and perspiration.
Depletion or excess of water in the body is often accompanied by depletion or
excess of sodium. Thus anything that leads to sodium depletion for instance,
may invariably lead to water depletion. Water depletion may result from
decreased intake as in coma, or increased loss as in severe sweating, diarrhea
in infants and cholera. Excess water may occur through increased intake as in
excessive administration of intravenous fluids, and decreased excretion as in
severe renal failure.
Maintenance of extracellular fluid between pH 7.35 and 7.45, and of which the
bicarbonate buffer system plays a key role, is essential for health.
Disturbances of acid – base balance are known (diagnosed) in the clinical
laboratory by measuring the pH of arterial blood and the CO2 content of
venous blood. Acid – base imbalance may either lead to acidosis (i.e. when the
blood pH is less than 7.35) or alkalosis (i.e. when the blood pH is greater than
7.45).
The causes of acidosis include diabetic ketoacidosis and lactic acidosis; and
causes of alkalosis include the vomiting of acidic gastric contents.
Homeostasis is a regulatory mechanism within a living organism that helps to
prevent water imbalance and acid-base disturbances.
ACIDOSIS
In (untreated) diabetes acetyl CoA accumulates resulting in the formation and accumulation of ketone bodies in the liver, which are acetone, acetoacetate and D-β- hydroxybutyrate. The utilization of these substances by extra-hepatic tissues such as the heart, skeletal muscle, kidney and the brain falls short of the rate of their accumulation in the blood, thus resulting in the net accumulation of these compounds in the blood. This lowers the pH of the blood causing the condition called acidosis. This type is called diabetic ketoacidosis.
Thus the above shows that untreated diabetes and starvation are conditions that promote gluconeogenesis, slows the TCA cycle by drawing off oxaloacetate, and enhances the conversion of acetyl CoA to ketone bodies
WATER IS THE IDEAL SOLVENT IN LIVING THINGS
Water is a slightly skewed (distorted) tetrahedral molecule. The tetrahedral structure is a three dimensional structure composed of an apex or centre and four base corners. The angle between any two corners is usually 109.5 degrees. However, the three dimensional structure of a molecule of water is an irregular tetrahedron with oxygen at its centre. The two bonds formed with hydrogen atoms are directed toward two corners of the tetrahedron, while the unshared electrons on the two sp3 – hybridized orbitals of oxygen occupy the two remaining corners. The angle between the two hydrogen atoms is slightly less than the normal tetrahedral angle. It is 105 degrees (fig 1.1)
Diagram
Key: i) the Bohr atomic structure of oxygen atom forming bonds with two hydrogen atoms.
Xx – are the two sp3 – hybridized orbital unshared electrons.
i. The skewed tetrahedron of water.
Water molecule is a dipole.
The dipolar nature of water arises from its skewed tetrahedral structure, which permits the unequal distribution of electrons (negative charge) in the structure between the oxygen atom and the two hydrogen atoms. The higher electronegativity of the oxygen atom in relation to the hydrogen atoms also contribute to the unequal charge distribution in the structure. The shared electrons spend more time on the side of the oxygen atom that is opposite the two hydrogen atoms. In other words, the electron density is higher on this side than on the side directly facing the two hydrogen atoms. Consequently, oxygen being electron-rich is slightly negatively charged, while the electron – poor hydrogen atoms have slight positive charge.
Besides water there are other biomolecules that form dipoles due to unequal charge distribution in their structure. Examples are ammonia, alcohols, phospholipids, amino acids and nucleic acids.
1.4 HYDROGEN BONDS IN WATER MOLECULES
The formation of hydrogen bonds in water to give rise to a macro-molecular arrangement or structure is a consequence of the dipolar nature of water. The ability of water dipolar nature to self-associate in both the solid and the liquid states make them exhibit macromolecular structure.
In water molecules a hydrogen bond is formed when electrostatic interaction occur between a hydrogen atom of one water dipole and the unshared electron pair of the oxygen atom of another water dipole.
The hydrogen bonds formed in liquid water are relatively transient in comparison to those of ice. Hydrogen bonds in liquid water have a half life for association – dissociation of about 1µs. In both liquid water and ice one water dipole is usually associated with four other water dipoles . However, strictly speaking, association of a central water dipole with four other water dipoles by hydrogen bonding is typical of ice (4.0), and to a lesser extent, of liquid water (3.5)
In comparison to covalent bonds such as the O-H bond of water, the hydrogen bond O---H is weak. It requires 110kcal/mole to break the O-H bond of a water molecule, but 4.5kcal/mole to bread the O---H bond between water molecules.
The dipolar nature of water and its ability to form hydrogen bonds explains why it can readily dissolve organic molecules. Examples of organic compounds that can form hydrogen bonds with water are amines, esters, aldehydes, ketones and proteins.
Macromolecules such as proteins that are stabilized by intermolecular hydrogen bonds may exchange such hydrogen bonds for hydrogen bonds to water thus enhancing its solubility. As a result soluble proteins appear coated with a layer of water.
1.5 DISSOCIATION OF WATER MOLECULES
Water dissociates slightly.
If one wishes to come closer to reality, one may depict the dissociation of water as an intermolecular proton (H+) transfer between two water molecules, forming a hydronium ion (H3o+) and a hydroxide ion (OH-).
But again the reality is something still a little bit different. The transferred proton is actually associated with a cluster of water molecules and exists in solution not even as H3o+ but as H5o2+ or H7o3+.
Thus in the simple equilibrium shown above the H+ is actually a highly hydrated ion. In the simple equilibrium dissociation of water, it cannot be known at any given time (t) whether an individual hydrogen or oxygen is present as an ion (H+ or OH-
Actually, the probability of a hydrogen atom in pure water existing as a hydrogen ion is about 1.8x10-9 (meaning that it has 1.8x10-9 chance in one of being an ion). And the probability of the hydrogen atom being part of a water molecule is almost unity (1) (meaning that there is an almost one chance in one of finding the hydrogen atom as part of a water molecule
The ionization of water can be mathematically represented as follows:
Where K is the dissociation constant, and the bracketed terms are the molar concentrations in mol/l of hydrogen ion, hydroxyl ion, and undissociated water molecules.
The value for the dissociation constant of water can be derived as follows.
The number of moles of a molecule can be expressed mathematically as
n = g/M
Where n = no of moles, g = measured weight (g) and M = molecular mass. So, 1 mole of water weighs 18g, and consequently 1000g (I litre) of water contains 1000/18moles
Pure water therefore has a molar concentration of 55.56 mol/l.
[H20] = 55.56 mol/l
Since the probability that a hydrogen in pure water will exist as an H+ ion is 1.8x 10-9, the molar concentration of H+ ions [H+] (or of [OH-]) in pure water is calculated by multiplying the probability by the molar concentration of water.
Ie, 55.56 mol/l H20 has a probability of I therefore for a probability of 1.8x10-9
Since water dissociates very slightly, the molar concentration of undissociated water (55.56 mol/l) remains approximately constant. The incorporation of this constant into the dissociation constant (K) provides a new constant called the ion product of water (Kw).
(K)[H2O] = [H+] [OH-]
Kw = (K)[H2O] = [H+] [OH-]
Kw = (K)[H2O] = [H+] [OH-]
Alternatively,
1.5 PH AND THE H+ CONCENTRATION
Sorensen defined pH in 1909 as the negative logarithm of hydrogen ion concentration
pH = -log[H+]
pH = -log[H+]
For pure water at 250c, PH = - log 10-7 = - (-7) = 7.0.
Low pH values mean high concentration of H+ ions and high acidity; but high pH values mean low concentration of H+ ions and low acidity.
It follows therefore that compounds that furnish a lot of hydrogen ions in solution are strongly acidic, while those that furnish low levels of H+ ions are weakly acidic. The quantity of H+ ions that a compound can release or donate in a solution depends on the degree of dissociation of that compound. Strong acids, for example, dissociate completely. Most inorganic acids are strong acids. Examples are hydrochloric acid (Hcl) and sulphuric acid (H2S04)
Most organic acids dissociate slightly or partially and are thus weak acids.
Examples are ethanoic acid and fatty acids
There are also strong bases and weak bases. Strong bases include potassium hydroxide (KOH) and sodium hydroxide (NaOH), but an example of a weak base is calcium hydroxide ca(OH)2.
1.6 PH CALCULATIONS
Example I: what is the pH of a solution whose H+ ion concentration is 3.2x10-4 mol/l
pH = -log[H+]
= -log (3.2 x 10-4) = -log3.2 + - log 10-4
= -log (3.2) - log (10-4)
= - 0.5 + 4.0 = 3.5
Example II: what is the pH of a solution whose OH-
POH = -log (OH-)
Kw = [H+][OH] = 10-14
log([H+][OH-]) = log10-14
log[H+] + log[OH-] = log10-14
= -0.60 + 4.0
= 3.4
pH = 14 - pOH = 14 - 3.4 = 10.6
Example III: what is the pH value of 2.0x10-6 mol/l KOH.
The OH-
i. KOH --- K+ + OH-
ii. H2O ---H+ + OH-
PH and POH are determined by the total [H+] concentration and [OH-
Sine KOH is a strong base ionizing completely [OH- ] = 2.0 x 10-6 mol/l. [OH-
Total [OH-
POH = -log(2.1 x 10-6)
POH = 5.7
PH = 14 - POH = 8.3
1.7 THE PHYSIOLOGIC SIGNIFICANCE OF WEAKLY ACIDIC FUNCTIONAL GROUPS
A lot of biochemical compounds possess functional groups that are weakly acidic or basic. Proteins possess carboxylic, amino and some other functional groups in their side chains that ionize in solution. Some metabolic intermediates possess the phosphoric acid group that ionizes strongly.
The dissociation behaviour of weakly acidic and weakly basic functional groups is very important to the structure and activity of metabolic intermediates. It is also very important to the separation and identification of biomolecules.
If the dissociation of a weak acid is represented as follows:
HA ---H+ + A-
HA (the protonated form of the acid) is the acid, and A- (the unprotonated form) is the conjugate base. And similarly, A- is the base and HA is the conjugate acid. For example, R-CH2 – COOH is a weak acid and R-CH2 – COO- is the conjugate base.
PK = -log K
The table below shows the K and PK values for a monocarboxylic, a dicarboxylic and a tricarboxylic acid
TABLE 1.0: DISSOCIATION CONSTANTS (K) AND PK VALUES FOR SOME CARBOXYLIC ACIDS
Acid | K | PK | Structure |
Acetic | 1.76x10-5 | 4.75 | CH3COOH |
Glutaric | 1st: 4.58x10-5 2nd: 3.89x10-6 | 4.34 5.41 | H I H2N-C-COOH (CH2)2 I COOH |
Citric | Ist: 8.40x10-4 2nd: 1.80x10-5 3rd: 4.00x10-6 | 3.08 4.74 5.40 | H I H - C-COOH I H - C-COOH I H - C-COOH I H |
THE LOWER THE PK VALUE OF A FUNCTIONAL GROUP THE HIGHER THE DEGREE OF DISSOCIATION. THEREFORE, THE STRONGER ACIDS HAVE LOWER PK VALUES
A weak acid has a strong conjugate base, and similarly, a strong base is a weak acid. Therefore the relative strengths of bases are expressed in terms of the pK (pKa) values of their conjugate acids. For example, the dissociation of the compound R-NH3+ to R-NH2 and H+ has a particular pK value.
It is known that the pK value for this dissociation is the same as the pH value at which the concentration of the acid (R-NH3+) equals that of the base (R-NH2). That is, if (R-NH2) = (R-NH3+), then the equation above simplifies to:
K = (H+)
So that – log K = - log [H+]
And thus, PK = PH.
Therefore, stated in other words,
the pK of an acid group is that pH at which the protonated and unprotonated species are present at equal concentrations
The pka for an acid can be determined experimentally by titrating (adding) 0.5 equivalent of alkali with 1.0 equivalent of acid, and the resulting pH will be equal to the pka of the acid.
Consider a weakly dissociating acid (HA):
The concentration of the undissociated acid (HA) in equilibrium is very high compared to that of the ionic species. If half of HA in equilibrium is reacted with equivalent base completely to furnish the conjugate base A- of approximately the same concentration as that of the remaining HA in solution.
i)
ii)
The pH of the equilibrium solution at this point is equal to the pKa of dissociation of HA.
ASSIGNMENTS
1. What roles do water and pH play in the structural and functional stability of biomolecules.
2. Discuss the homeostatic regulation of water and pH in the body fluids of organisms.
3. What is acidosis? Discuss.
4. Discuss water under the following headings:
a) Structure of water
b) Water as a dipole
5. What is the pH value of 2.0 x 10-6 mol/L KOH
6. Derive the Henderson-Hasselbalch equation for a weakly ionizing acid (HA)
7. What is the pH of a solution whose OH-
8. What is the pH of a solution whose H+ ion concentration is 3.2 x 10-4 mol/L
9. Discuss Sorenson’s definition of pH.
10. Explain why and how water forms hydrogen bonds.










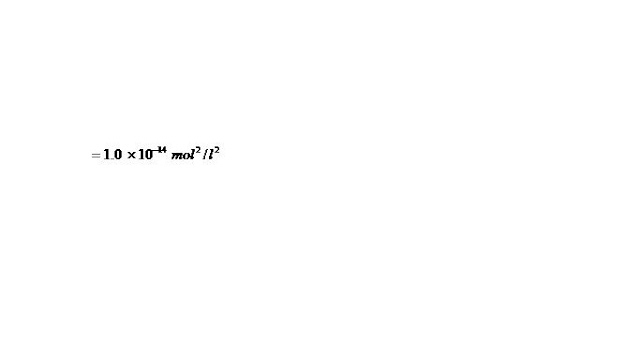








No comments:
Post a Comment